Silver Plating of Copper or Copper Alloys – Silver Properties
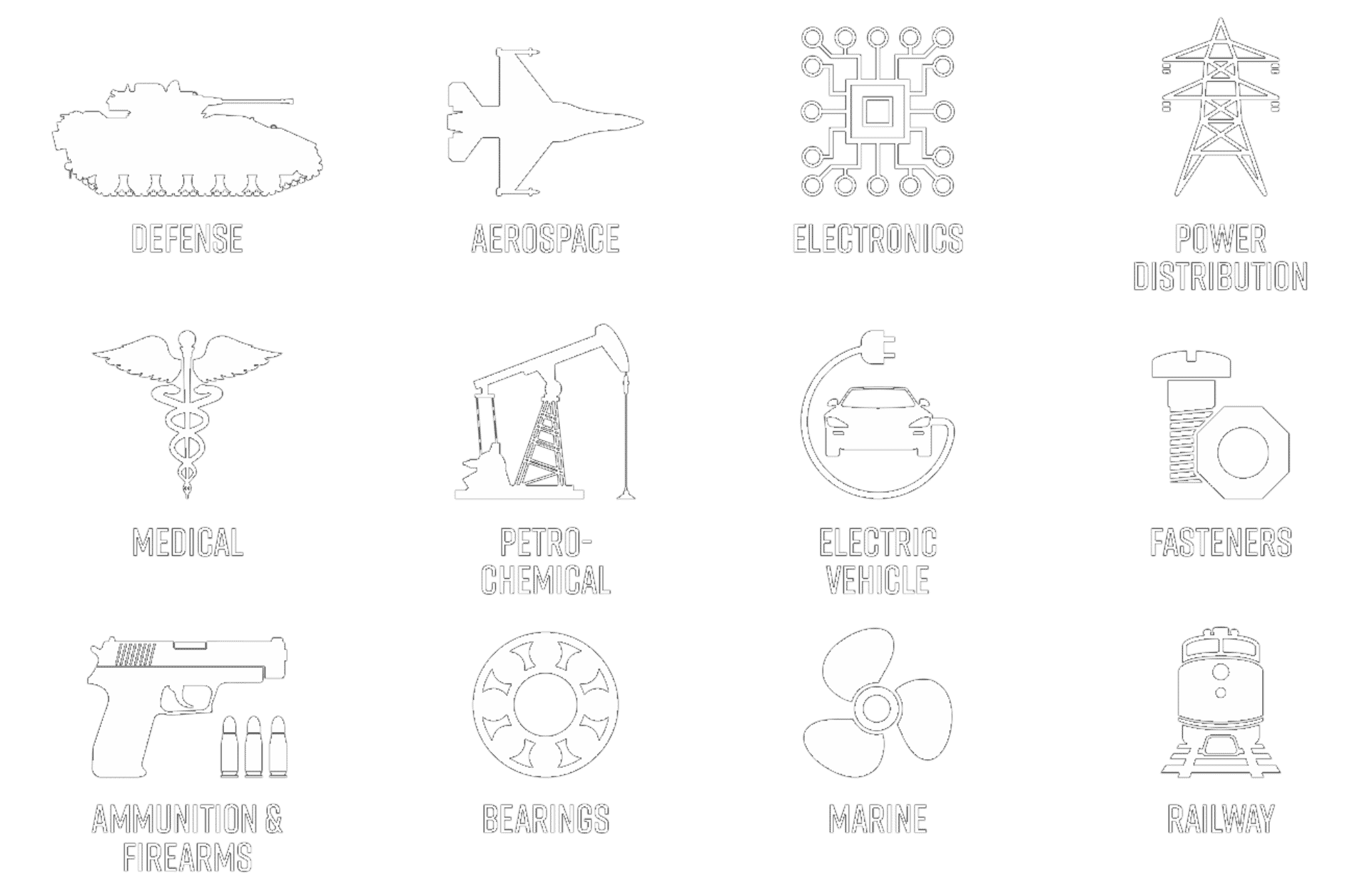
Silver plating of copper or copper alloys is a highly functional finish for transferring heat and electricity utilized across a wide breath of industries. Silver has been applied since late 1800s on electrical switchgear and other components that pass electrical current. In recent years silver plating of copper electronic components including connectors and terminals has grown rapidly within the electronic, automotive and electric vehicle (EV) markets. Silver plating has many unique properties that make it desirable for these applications. The primary reason is that silver has the highest electrical and thermal conductivity of any metal, which facilitates the efficient transmission of electricity and heat. In addition, silver is a relatively soft metal which allows the silver deposit to compress and form around a mating connecter filling small voids and micro-roughness. This increases the effective contact area resulting in less overall connector resistance.
Silver has excellent lubricity and resists galling in switching, sliding or rotary applications. However, high-pressure wear surfaces such as blade-style stab connectors can be susceptible to silverwear. In applications such as this, a higher deposit thickness of silver is recommended as well as the use of a nickel or electroless nickel underplate. Thinner silver plating without a nickel underplate is best used on static joints or low duty cycle connectors that are mated and unmated relatively infrequently.
One of the historical concerns with silver plating is the formation of silver sulfide compounds, commonly called silver tarnish. The tarnishing of silver is primarily a cosmetic concern since it produces only a relatively small reduction in conductivity. However, for micro-electronics and low-voltage connectors, the potential for tarnish should be accounted for with proper selection of an anti-tarnish inhibit. There are many different anti-tarnish inhibitors available to avoid silver tarnish that perform very well including organic, thiol based and tin immersion systems.
Copper Properties
Copper materials have been the work-horse material within the electrical and electronic industries for more than a century. This is because, like silver, copper also has many desirable properties such as excellent electrical and thermal conductivity, which makes it great for handling high voltages and high currents. Copper is also a relatively noble metal which reduces the potential for corrosion when in contact with metals such as tin, nickel or silver. For this reason, silver plating of copper provides many design benefits since the properties of the two metals complement each other. Numerous copper alloys are available today which provide a range of mechanical and electrical properties. Many of the common grades of copper used in manufacturing today include C101 (Oxygen Free), C110 (ETP – Electrolytic Tough Pitch), C145 (Tellurium), C147 (Sulfur Bearing), C172 (Beryllium), and C182 (Chromium). All these different grades of copper can be silver plated to increase the electrical performance in its designated application.
Benefits of Nickel Underplating
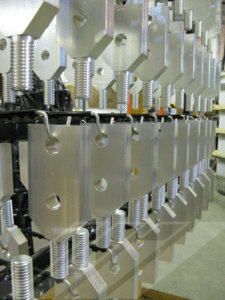
The use of a nickel underplate prior to silver plating copper can improve the performance of the overall deposit in several ways. A few of the benefits of nickel underplate are summarized below:
- Diffusion barrier: When silver is plated on copper a natural solid-state diffusion of the copper into the silver will occur over time. The rate of diffusion is accelerated greatly at elevated temperatures. As copper diffuses into silver it forms a hybrid copper/silver eutectic layer which negatively affects adhesion, solderability and electrical performance. Nickel forms an effective diffusion barrier to prevent this copper/silver migration from occurring. This intermediate layer of nickel is highly recommended in most specifications and even required in some such as ASTM B700 (Section 6.3.4) and QQ-S-365 (Section 3.3.5).
- Corrosion Performance: Silver protects copper from oxidation by forming a barrier layer between the environment and the copper substrate; this type of corrosion protection is commonly referred to as barrier corrosion protection. The addition of a nickel underplate prior to silver plating helps form a more effective barrier between the copper base and the environment since it limits the overall deposit porosity. Since nickel is a less expensive metal than silver, a heavy deposit of nickel can be used to improve corrosion performance with much less cost impact than increasing the silver thickness alone.
- Load/Bearing Durability: The use of a nickel underplate helps provide a rigid structure for which the silver layer can be deposited upon. This helps reduce the wear of the silver in high contact pressure switching and wear applications such as fuse stabs or high voltage switch gear.
- Solder Base: The use of a proper nickel underplate prior to silver plating can help improve the solderability of a copper component; this is especially true if soldering copper alloys. In addition, since the nickel helps reduce the diffusion of copper into the silver, a nickel underplate can extend the shelf-life of solderability and reduce the overall amount of silver that needs to be plated to successfully solder a copper components such as busbars or terminals.
The most common nickels used as an underplate to silver are an electrolytic sulfamate nickel or electroless nickel. The selection of the type of nickel underplate depend on many factors including part geometry, design corrosion performance, cost, solderability and even magnetic concerns. A member of Advanced Plating Technologies’ engineering or technical sales staff can help assist with selecting the best nickel underplate as well as recommended deposit thickness.
Silver Plating of Copper or Copper Alloys – Plating Cycle and Pre-treatment Considerations
In silver electroplating of copper alloys, small differences in the alloy composition can have a large impact the plating cycle to ensure a high-quality final silver deposit. Even though most copper alloys have a majority of their composition made from copper, small percentages of alloying elements can require specific pre-treatment or strike layers to ensure the final silver layer is adherent. A brief summary of some of these factors are listed below for many common copper and copper alloys.
Silver Plating of C101 (Oxygen Free) and C110 (ETP) Copper
Copper alloys C101 and C110 are the most straightforward copper grades to plate being 99.9% or more pure copper. For these two alloys it is recommended that they both receive an alkaline cleaning pretreatment to clean the parts as well as an acid pickle to remove any oxides from the copper surface. This results in an “active” copper surface that is receptive to receive the subsequent plating steps. The copper can then receive a nickel underplate (if specified) followed by a silver strike and finally the silver plating. The silver strike layer is very important to achieve an adherent silver layer and avoid silver immersion that would result if the copper was immediately silver plated without a strike.
The above steps will be referred to as the standard method, because this method usually only needs to be altered slightly to accommodate for the other copper alloys.
Silver Plating of C145 (Tellurium) Copper
Tellurium copper is a highly machinable alloy of copper with excellent electrical properties. For this reason, tellurium copper is one of the most commonly used copper alloys when the component is manufactured by machining operations such as turning or milling. Tellurium copper requires a unique pretreatment due to the 0.5% tellurium in the alloy. Because of the tellurium this material cannot be exposed to a cyanide plating solutions. If this occurs an insoluble tellurium/cyanide compound results that will not be receptive to subsequent plating. Since most silver strike and silver plating baths are cyanide formulations; tellurium copper must first be underplated with a non-cyanide process such as nickel, acid copper or tin. Once underplated in this manner, the rest of the pretreatment process is the same as the standard method.
Due to the need for an acid-based strike prior to silver plating, alternative machinable coppers such as C147 sulfur copper may be preferred to C145 tellurium copper in certain applications. These would include specifically applications with deep, ID features that may be difficult to fully plate with the acid-based strike without affecting part dimensions and tolerances. Advanced Plating Technologies’ sales and engineering staff can help provide input to assist with design for manufacturability to ensure the best material is selected from a finishing standpoint.
Silver Plating of C147 (Sulfur) Copper
C147 Copper is essentially pure copper with only about 0.4% sulfur added. However, this small addition of sulfur gives C147 much improved machinability over that of C101 or C110 copper. In fact, C147 copper has the same machinability rating as that of C145 tellurium copper (85% of free-cutting brass)2. From a plating perspective, C147 copper is much preferred over C145 copper since there is no tellurium in the alloy. Since C147 is tellurium free, it can be pretreated and plated without the need for an acid strike consistent with the standard method noted above. As such, C147 can offer considerable cost savings over that of C145 tellurium copper for silver plating; this is especially true if parts have complex geometries.
Silver Plating of C172 and C173 (Beryllium) Copper
Beryllium copper, much like tellurium copper, gets a special pretreatment because of the 1.9% beryllium that it contains. Because of the beryllium content the surface of this copper alloy tends to have beryllium oxide that must be removed before plating the part. In addition, C172 and C173 copper are often heat treated prior to plating resulting in a heat treat scale that must be removed prior to plating. The most common method to remove the layer of oxides/scale is a bright dip process (a mixture of several acids). A proper bright dip will remove the oxide layer and beryllium-rich regions leaving an active copper surface. After bright dipping beryllium copper, the standard plating method can typically be used. Since a bright dip operation is typically performed off-line and not in-line with a typical plating process; silver plating of beryllium copper alloys can be more expensive than other copper alloys.
Silver Plating of C182 (Chromium) Copper
Chromium copper is alloyed with a small percentage of chromium (~ 0.8%). This seemingly small addition of chromium makes this copper alloy able to be heat treated and improves both the strength and hardness. The addition of chromium necessitates the use of a specific electrolytic nickel activator after the initial cleaning and acid pickling. After activating the material, the standard copper plating methods are followed. The nickel activator that is used is a very inefficient process which does not reach within small ID features of a part well. As such, C182 chromium copper parts with complex geometry can pose unique challenges to activate prior to silver plating.
Silver Plating of C260 (Cartridge) Brass
C260 cartridge brass can be considered the basic form of brass since it is a combination of copper and zinc only. Zinc usually makes up about 30% of the material’s composition with the remainder being copper. C260 brass is very popular as it offers good corrosion resistance and the highest ductility of the yellow brasses. C260 brass is commonly used for manufacturing components that are stamped or drawn. Even though this alloy has a significant difference in composition, C260 brass is pretreated very similarly as C102 and C110 Copper. However, care must be taken in the activation of the alloy not to overclean the material as the zinc in the alloy can react. Other than this modification, the basic standard plating method works well for silver plating of C260 brass.
Silver Plating of C360 (Free Machining) Brass
C360 Brass is different from C260 for several reasons. The first is that the zinc content is higher (about 37%) and the second is that it also contains lead (about 3%). The addition of the lead helps make C360 brass one of the most machinable copper alloys available and is commonly used for turning and milling applications. A side effect of the addition of lead is that it requires modification to the pretreatment for this alloy. A duplex acid pickle must be used in which the first acid removes the standard copper/zinc oxides from the surface and the second alloy removes any lead inclusions present on the surface. As with C260 brass, care must be taken not to overclean the brass since the zinc in the substrate makes the alloy more delicate than a pure copper alloy. Beyond these modifications, the standard method applies to silver plating C360 brass.
Silver Plating of Copper or Copper Alloys – Thickness of Silver Plating
Silver plating of copper or copper alloys usually falls within one of four thickness ranges: flash, commercial, moderate, severe or bearing applications. Each of these different categories uses a different range of thicknesses that are necessary for the plating of the part to meet design requirements.
| Silver Category | Silver Thickness | Description |
| Flash | 0.00005 inches (1.25um) | Very light duty, no wear or corrosion concerns |
| Commercial | 0.0001-0.0003 inches (2.5-7.5um) | Light duty connectors and contacts with limited wear and corrosion resistance for mild interior applications only |
| Moderate | 0.0003-0.0008 inches (7.5-20uin) | Moderate duty connectors with higher switching and contact loads; moderate exterior corrosion applications |
| Severe | 0.0008-0.002 inches (20-50um) | High wear with heavy contact loads; high corrosion environment including exposure to chemical attack |
| Bearing | > 0.002 inches (> 50um) | High pressure/load bearings in petrochemical, heavy equipment or aerospace applications |
Silver Plating of Copper or Copper Alloys – Barrel vs Rack Plating Method
One of the largest factors impacting the cost of silver plating is whether a component must be barrel or rack plated. These two methods are the most common way to plate “loose piece” copper components. In barrel plating parts are mass loaded into a vessel that rotates during processing while passing current into the load. In rack processing, the components are individually fixtured on a framework that passes current into the parts. Barrel plating offers considerable labor savings since the parts can be handled in mass which makes this plating method a much more cost-effective way to silver plate smaller copper components. However, the parts must be smaller and amendable to tumbling which limits the size and type of component that can be barrel plated.
Barrel plating can be performed with a variety of barrel sizes and configurations. Small barrels can be 2” in diameter and 4” long whereas larger barrels can be as large as 14” in diameter and 48” long. The selection of the barrel will depend on the part size/configuration and how much product is to be plated. Barrel plating only works if you have enough volume of parts to fill the vessel between about 30-50%. For this reason, if parts are quoted with a barrel plating method, it is typical to list a MOQ (minimum order quantity) in order to successfully barrel plate.
The ability to barrel silver plate a copper component depends on three primary variables: the size of the part, the weight of the part and surface finish requirements. Typically, parts must be less than 3” in any dimension and less than 0.75” in diameter as a very general rule. Most commonly, copper components must be less than approximately 0.1lbs to barrel plate; however, the part geometry matters as well. For example, soft copper parts with exposed male threads can be damaged during barrel plating. In addition, parts that can interlock, stick together or nest during tumbling often cannot be barrel plated. The final consideration is the surface finish of the part. Since parts are tumbled during barrel plating, highly machined surface finishes (< 30uin) can be increased by the tumbling action of barrel plating. Again, the part size/weight must also be considered when reviewing surface finish as smaller parts with a low surface finish are more likely to be successfully barrel plated than larger copper parts.
For copper parts that cannot be barrel plated, rack plating is a viable alternative. Parts are usually rack plated because they are either too large for barrel plating or the parts might nest/interlock or otherwise become damaged while tumbling. One consideration when rack plating is that a rack mark or lack of full plating coverage will result where the part is contacted by the plating fixture. There are ways to minimize this contact point; however, this needs to be discussed during the development phase of a new application. Although rack plating is higher cost process than barrel plating, it can be used to successfully plate very large parts of nearly any geometry of configuration
Silver Plating of Copper and Copper Alloys – Conclusion
Silver plating of copper and copper alloys produces a highly conductive, lubricious, and corrosion-resistant coating utilized throughout the Electric Vehicle Market (EV) and similar markets. The combination of a silver coating on copper provides a surface that is highly conductive both thermally and electrically making this combination an excellent choice for parts that transfer heat or electricity. The diversity of copper alloys offers a broad range of mechanical properties which increases the applications where copper can be utilized. The alloys of copper must be pretreated and activated in unique ways which can have an impact in the methods and cost of silver plating. Proper selection of underplates and silver thickness are critical for the function of a silver plated copper component to ensure the application meets the intended service life. Finally, the size, geometry and surface finish requirements of a copper component can impact whether it can be either barrel or rack silver plated.
For more information on silver plating of copper or other applications, please contact a member of Advanced Plating Technologies’ engineering or technical sales staff.
Blog Authored By: Dominic Scardino, Estimating Engineer
References:
- Maglione, Gregorio. Exploring the Realm of Conductivity. Digital Image. https://ysjournal.com/exploring-the-realm-of-conductivity/ Young Scientists Journal. 22 April 2017 Published. 28 April 2020 Accessed.
- Oberg, Jones, Horton, and Ryffell. Machinery’s Handbook, 26th Edition, New York. Industrial Press Inc. 2000. Page 533-534.
- Crimp Connectors by Burndy. Digital Image. https://www.farwestcorrosion.com/crimp-connector.html Farwest Corrosion Control Company. 28 April 2020 Accessed
- Sterling Systems Products for Platers, Page 7. Digital Image. http://www.sterlingsystems.com/sterling.pdf Sterling Systems. 28 April 2020 Accessed