Electroplating is among the most critical processes used in modern manufacturing today. With the Internet of Things (IoT) more and more devices are connected to provide instant access to information and data. However, any electrical device’s effectiveness and reliability depends highly upon the quality of the connections and specifically the plating used on the connector or contacts. Proper electrodeposition is key to ensuring a device performs reliably whereas improper plating can negatively affect performance, utility and durability of the electrical device.
When it comes to plating electrical components such as connectors and contacts, gold and silver are two common electroplating processes that produce highly reliable and conductive connections. However, there are advantages and disadvantages between silver and gold plating services. Both gold and silver are highly conductive and corrosion resistant; however, silver will form a sulfide compound (tarnish) and gold can be a costly option. In this article, we’ll be discussing the difference between gold and silver connectors and when one coating might be preferable over the other.
The Benefits of Gold-Plated Connectors
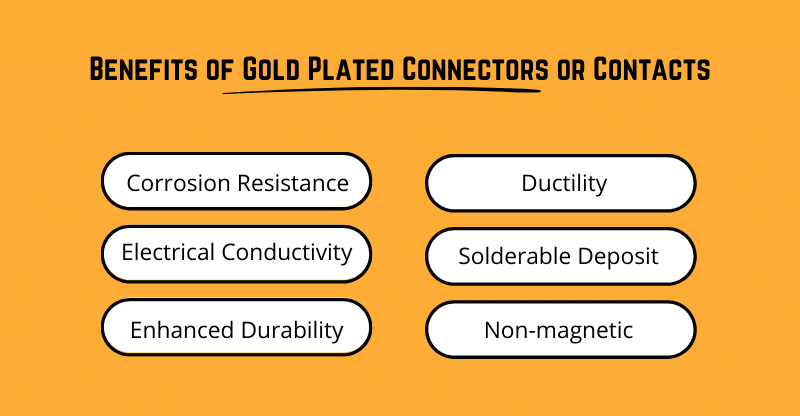
Gold is a highly noble (unreactive) metal that can enhance the performance of connectors in a variety of electrical applications. The benefits of using gold-plating include:
Superior Corrosion Resistance
In comparison with other metals, gold’s resistance to oxidation or corrosion is extremely high. In situations where the contacts of a connector are likely to be exposed to corrosive substances or conditions, gold plating can serve as an effective barrier from oxidation and corrosion. As a result, gold-plated connectors are an excellent choice for more corrosive applications where the connector or contact may be exposed.
High Electrical Conductivity
Besides copper and silver, gold is the third most conductive metal in the world. However, gold does not produce any oxides or other compounds, so it maintains its high conductivity even at elevated temperatures or when exposed to corrosive environments. The high/consistent conductivity of gold ensures stable current flow even at very low voltages making gold an excellent choice for electronic applications where milli-volts transmit milli-amps.
Enhanced Durability
Electroplated gold can be alloyed with small amounts of nickel or cobalt to increase the hardness from that of pure gold (< 90 Knoop) to as high as 200 Knoop. This hardened gold deposit is commonly referred to as hard gold. When plated to a sufficient thickness (> 50uin) over an electrolytic or electroless nickel base, hard gold can provide a durable and coating for repeated connection cycles. Hard gold is not prone to fretting or galling due to its natural lubricity.
Ductility
Because gold is such a malleable metal, it’s suitable for flexible connections and springs. Gold’s ductility makes it more likely that plating will hold up to multiple cycles of contact. However, gold-plated electrical connectors or springs require a suitable underplate material to guarantee the finish meets the design requirements. It is generally recommended that an engineered nickel such as a sulfamate nickel be used as an underplate to gold when a plating a flexible contact or spring.
Solderable Deposit
Gold plating is an excellent finish for forming reliable solder joints and will consistently and evenly wet with using just a mild rosin flux without acid activation. Gold can be plated on nearly any substrate including stainless steel terminals or connectors to allow for subsequent joining through soldering. Generally, only a thin deposit of soft gold of 0.00001 inches per side is required to form a reliable solderable gold contact but heavier gold deposits can be soldered as well.
When soldering to a gold electrodeposit, the gold plating diffuses into the solder joint through a mechanism called solid-state diffusion. Because of this phenomenon, care should be taken to not exceed more than 3% by weight gold in the solder joint as this can cause embrittlement within the joint itself. As a general rule deposits of < 0.00005 inches per side will result in less than 3% by weight gold in the solder joint
Non-magnetic
As a final point, gold is not magnetic. This is advantageous in scenarios where electromagnetic fields can create interference. For instance, gold plating may be suitable for connectors used in medical equipment like Magnetic Resonance Imaging (MRI) scanners.
Industry Applications of Gold-Plated Connectors or Contacts
Most the electronic devices we rely on each day utilize gold-plated contacts or terminals. In addition to gold’s attractive, value-added appearance, this precious metal has several key properties that make it a valuable material across many industries. However, the electronics and interconnect industries are the primary user of gold. It performs an essential function in keeping electronic components working effectively over time.
Gold can be found in various electrical devices, including cell phones, desktops, and laptops. For every 10,000 smartphones, there are about ten troy ounces (or 3/5 pounds) of gold. There are approximately five troy ounces of gold worth over $9,000 in 200 computers.
Because of its capacity to maintain electrical connectivity, gold is well-suited for use in a wide range of electronic applications. It can be applied to any part of a device that requires a reliable electrical connection. External components such as electrical connectors most commonly feature gold plating. However, gold is primarily used in the circuit boards of electronic devices.
The reliability of any device is dependent on the integrity of its circuit board connections. As such, electronics manufacturers apply gold plating to their circuit boards to improve conductivity and prevent corrosion. 99.9% pure gold plating or soft gold, as it is called, is commonly used in pad connections or where wire bonding is required.
The Benefits of Silver-Plated Connectors or Contacts
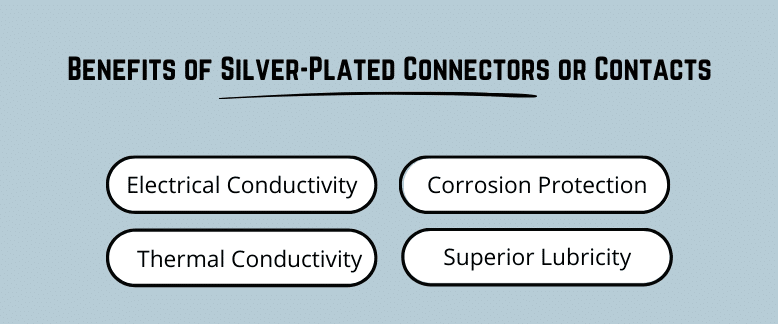
Similar to gold, silver is a precious metal that provides a very conductive surface while forming an effective barrier to corrosion. The primary advantage that silver provides is it is about one one-hundredth the cost of gold which allows for a wider range of use and plating at higher thicknesses than gold. The primary disadvantage of silver is that silver is a semi-precious metal and forms sulfur compounds such as silver sulfide or tarnish which can affect the conductivity of the silver electrodeposit over time.
Highest Electrical and Thermal Conductivity
Because of its high conductivity and lower cost, silver is perhaps the ideal precious metal for applications where high power transfer of electricity is a design requirement. Examples include plating of current exchange bodies, fuse cutouts, stabs, terminals bus bars or other high power connectors. Because of the lower cost, plating of larger copper or aluminum conductors with silver is not cost prohibitive and provides a very reliable precious metal coating that resists oxidation and produces low contact resistance.
Aside from electricity, silver is also an excellent conductor of heat, which allows connections that transfer large amounts of power to naturally regulate thermal hot spots preventing oxidation of the substrate materials.
Protection Against Corrosion
Like gold, silver is a precious metal that can form an effective barrier to corrosion. Because of the lower cost, silver can commonly be plated to thicknesses exceeding 0.001” per side forming a very pore-free precious metal barrier to corrosion. Silver is commonly plated over an electrolytic or electroless nickel barrier further increasing the overall corrosion protection.
When plated over a copper or aluminum conductors, silver forms an effective barrier to prevent the substrate materials from forming compounds or oxides. This eliminates increase in contact resistance or thermal hot spots in a conductor over time.
Superior Lubricity
Silver is a natural metallic lubricant which provides outstanding lubricity even at extreme temperatures. Silver is an excellent choice for applications such as high temperature threaded or sliding contacts where galling may be a design concern. Silver is commonly used for plating of stainless steel and other high temperature alloys to preventing seizing of moving or threaded parts within turbine engines or turbo chargers where temperatures can exceed 1250°F.
Silver provides outstanding sliding lubricity on high contact pressure switches or contacts including fuse pad or stab connectors or high pressure socket connectors. The use of a lubricous nickel underplate such as electroless nickel can further enhance the lubricity and wear properties of silver electroplating on a connector or contact.
Industry Applications of Silver-Plated Connectors or Contacts
In industrial settings, silver plating’s outstanding electrical and lubricating properties are commonly used in switching electrical power applications. Because of its relatively low cost and good conductivity, excellent lubricity and corrosion resistance, silver plating is suitable for a wide range of applications. There are more applications for silver electroplating than any other plated precious metal. This is largely due to silver’s low-cost relative to the other precious metals such as gold, platinum, palladium or rhodium.
Silver plating is widely employed across a range of sectors and in a broad array of electrical products and applications, including:
● Power Transmission and Distribution: bus bars, relays, current exchangers, reclosers, fuse tabs, stab connectors, power connectors, disconnects
● Electric Vehicle Market: stationary and movable connectors, relays, power invertors, bus bars, weld pads to facilitate ultrasonic welding, charging connector pins and sockets
● Electronics: terminal pins and sockets, solder pads, power lugs
● Bearing & Transmission: thrust washers and high temperature bearings, corrosion resistant fasteners and lock nuts for turbine engines and turbochargers
The growing use of electrical power in applications such as electric vehicles and electronics make the future demand of silver very high. It is projected that the demand for silver plating will continue to grow exponentially over the upcoming years.
What Underplates Are Used for Gold vs Silver Plated Connectors or Contacts?
Prior to plating silver or gold, it is common to use an underplate of another metal. Underplates can provide improved corrosion resistance, create a structural base for the subsequent precious metals deposit, improve overall conductivity and help prevent diffusion of elements such as zinc from the substrate into the ultimate precious metals deposit. A few examples of metals used as underplates prior to gold or silver plating include:
● Copper: Copper plating is an excellent underplate metal for effectively sealing the base substrate to promote adhesion, conductivity and corrosion protection. It can be used to coat a wide range of metals and promotes cleaning of the substrate as it plates due to its cyanide-based formulation. Being an excellent electrical and thermal conductor, copper can help promote the current carrying capacity of a conductor and mitigate hot spots in connections. Copper is also a very ductile underplate making it suitable for springs or crimp-style connectors.
● Electrolytic Nickel: When gold or silver plating copper or copper alloys such as brass, substrate elements including copper and zinc can migrate into the gold or silver deposit over time to form an intermediate eutectic layer. This eutectic decreases the adhesion, conductivity and solderability of the gold or silver layer. An electrolytic nickel underplate forms an effective diffusion barrier between the substrate and the gold or silver ultimate layers.
Additionally, electrolytic nickel plating provides a firm, structural base for subsequent gold or silver plating to promote wear resistance and helps provide an increased barrier to corrosion. Certain electrolytic nickels such as sulfamate nickel provide good ductility and can be used for spring contacts or crimp applications. Finally, electrolytic nickel has a very high melting point over 2000°F, making it a good underplate selection for high-temperature applications.
● Electroless nickel: Electroless nickel plating provides many of the above benefits of electrolytic nickel including diffusion barrier, structural base and improve corrosion resistance. However, electroless nickel has the added benefit of being extremely uniform and as such, can be plated to high thicknesses without negatively impacting the dimensional tolerances of a part.
Electroless nickel also has improved lubricity and hardness over that of traditional electrolytic nickel and high phosphorous electroless nickel is one of the few non-magnetic nickels to use as an underplate. Electroless nickel does have a significantly suppressed melting point of 1500°F and as such, it should not be used in extreme high temperature applications. Finally, electroless nickel does not have as high of ductility as electrolytic nickels such as sulfamate nickel and as such, is not recommended for crimp applications.
Gold vs. Silver Connectors or Contacts: What Are the Differences?
Gold and silver are among the two most frequently used precious metals for connectors and contacts in various sectors. There are advantages of each but there are also disadvantages and differences between these two popular finishes. Here are a few major differences between gold and silver electroplating.
Cost – A Gold Plating Disadvantage
Global industrial demand, political and economic uncertainty, and currency devaluation all play a role in driving up the price of gold. Many countries and people turn to gold in periods of economic instability because of its globally accepted value as a currency alternative. However, with the IOT, gold is now sought for more industrial reasons than merely as an investment or as decorative jewelry: gold is truly an essential metal in the production of modern electrical and electronic devices.
Increasing gold prices can significantly affect the manufacture of gold-plated components, especially for applications which use heavy gold deposits. Even though no other material can match all the properties of gold, silver has many similar properties at a significantly reduced price. Silver can be plated more heavily and at a lower cost with deposit that yield many similar properties. However, the formation of sulfide compounds or silver tarnish, is one of the limiting factors for silver in applications very sensitive to increases in contact resistance.
Silver Tarnish – A Silver Plating Disadvantage
Silver does not form oxides or compounds with oxygen under normal conditions; however, silver plating does form various sulfur compounds such as silver sulfide. Although silver sulfide compounds are relatively conductive, they do increase the contact resistance of the silver plating over that of pure silver alone. In many switching applications, any silver tarnish is effectively wiped from the surface within the sliding contact zone. However, in static applications, silver sulfide or tarnish can increase the contact resistance enough to change the signal path for very low voltage applications.
There are various anti-tarnish inhibits such as Enthone’s Evabrite products or Technic’s Tarniban products; however, all of these anti-tarnish compounds do add an organic or metallic film on the surface that alters the properties of the silver electrodeposit away from that of pure silver.
In contrast to silver, gold does not form sulfide compounds or tarnish under any normal condition. That makes gold a more viable option for lower voltage signal transmission applications where minor changes in contact resistance can impact product performance. Critical applications such as life-safety sensors or applications for autonomous vehicles require extremely reliable real-time signal transmission that only gold plating can provide.
Conductivity – Silver Vs Gold Electroplating
Silver is more conductive electrodeposit than gold. However, gold’s ability to not form any resistive compounds make it ideal for milli-amp data applications. It is also a good choice for low-voltage applications and corrosive conditions. On the other hand, silver’s superior heat and electrical conductivity and ability to plate to higher thicknesses cost effectively, make it the preferred material or high voltage and high current power transmission applications.
Why Use Gold Electroplating Services for Your Connectors?
While gold possesses several qualities that make it suitable for electronic components, there are several key attributes that need to be considered when specifying gold plating for a connector or contact application. Here are a few key factors to consider when specifying gold plating services for a new application:
Gold Plating Services – Ensure Proper Plating Thickness
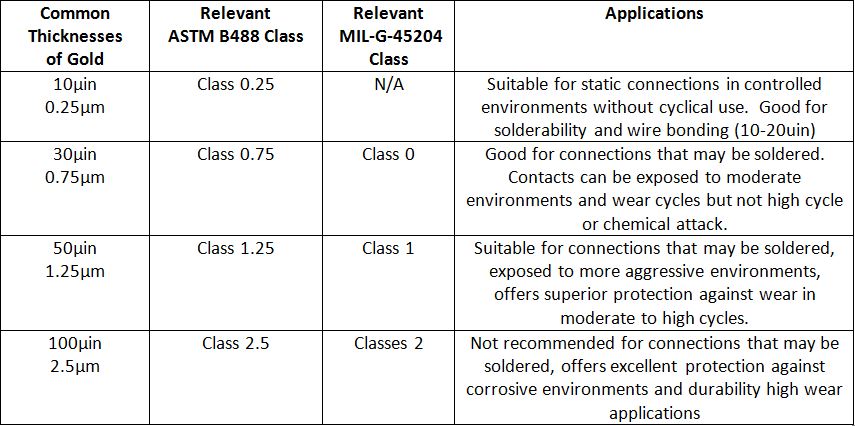
When specifying gold plating it is important to callout sufficient gold thickness to ensure proper function without calling for excess gold. The table below provides some basic guidance for the proper thickness of gold plating for most connectors and contacts.
As a general rule, functional gold begins at approximately 0.25 microns or 0.00001 inches and increases to as high as 2.5 microns or 0.0001 inches per side. The use of duplex gold or two-layers of soft followed by hard gold can provide a more effective barrier deposit of gold per mil thickness than with a single layer alone.
Gold Plating Services – Specify a Proper Underplate
As noted above, gold plating is commonly proceeded by an underplate of copper and/or copper and nickel. The underplate selection and thickness are just as important as the final gold thickness to ensure a deposit that provide sufficient durability, corrosion resistance, ductility and solderability. Copper plating helps promote adhesion and provide improved corrosion resistance and conductivity. Whereas a nickel underplate provides an excellent diffusion barrier to prevent solid state diffusion of copper or alloying elements such as zinc from forming a eutectic between the gold and the substrate.
Nickel plating also promotes corrosion performance and wear resistance of the gold by provide a hard foundational structure for which the gold can be subsequently plated on.
Gold Electroplating – Hard vs Soft Gold
As-plated 99.9% minimum plated gold is a soft deposit with a maximum hardness of 90 Knoop 25. This deposit is best suited for static, low pressure contacts (50-gram load or less) or applications that will be wire bonded or soldered. By alloying a small amount of nickel or cobalt with the gold, the hardness can be increased to as high as 200 Knoop 25 providing much improved wear properties.
These hard-gold deposits are generally recommended for higher contact pressure applications (greater than 50-gram loads) or applications with sliding wear such as female/male contact pins. More information can be found at the following link on the differences between hard gold plating vs soft gold plating.
The Bottom-line
Selecting the appropriate plating for connectors and contacts is a key part of manufacturing a reliable product. The type of electrodeposit used can impact the product’s quality, performance, longevity, and cost. Gold and silver are both high-quality precious metal deposits, but they have different unique benefits and drawbacks that should be considered prior to specifying a specific finish. This article has covered the foundational principles to consider between gold and silver plating.
The above information is provided as a general guide when considering silver plating verses gold plating for a connector or contact application. There are many additional considerations specific to each design that are beyond the scope of this article. Advanced Plating Technologies offers extensive surface engineering support for gold plating and silver plating services.
Reverse engineering of existing or failed applications and components is available to provide technical design assistance. Feel free to contact a member of APT’s technical sales team for further assistance at [email protected] or 414.271.8138.