Nitric vs Citric Passivation Methods
Stainless steel is an inherently corrosion resistant material, however when stainless steel is machined, formed or fabricated free iron can be introduced to the surface that can corrode independent of the base material. Proper passivation of stainless steel with an oxidizing acid such as nitric or citric acid removes this free iron and promotes the growth of a thin, dense protective oxide layer which maximizes the corrosion resistance of the stainless steel. Depending on the type of stainless steel and end application certain passivation processes may perform better at passivating than others. In this article we will compare nitric vs citric acid passivation which are the two primary chemistries specified in ASTM A967 and AMS 2700.
Nitric Acid Passivation
When comparing nitric vs citric passivation, the most common method used throughout industry is nitric acid passivation. The Nitric acid passivation processes was the original passivation processed specified in QQ-P-35, the first military specification covering passivation, revision A being released in the 1960s. Nitric acid passivation offers a range of options to customize the oxidizing potential of the acid to suit a specific grade of stainless steel. The various methods and types of nitric acid passivation include several heated options as well as options that include a sodium dichromate.
The higher nitric acid concentration and the higher the nitric acid temperature, the more oxidizing potential the passivation chemistry has. Sodium dichromate can also be added to the nitric acid to increase the oxidizing ability of the bath making it better for less corrosion resistant stainless steels, such as precipitation hardened, martensitic and ferritic grades of stainless steel. These grades of stainless steel have less nickel and chromium in them making them more susceptible to etching. The higher the oxidizing potential of the chemistry, the faster and more effective the passive oxide barrier is formed on the surface, reducing the potential for etching.
A summary of the various nitric acid passivation methods per ASTM A967 is provided below:
- Nitric 1: 20-25 v% Nitric Acid, 2.5 w% Sodium Dichromate, 120-130F, 20 Mins minimum
- Nitric 2: 20-45 v% Nitric Acid, 70-90F, 30 Mins minimum
- Nitric 3: 20-25 v% Nitric Acid, 120-140F, 20 Mins minimum
- Nitric 4: 45-55 v% Nitric Acid, 120-130F, 30 Mins minimum
- Nitric 5: Other combinations of temperature, time, and acid with or without accelerants, inhibitors or proprietary solutions capable of producing parts that pass the specified test requirements
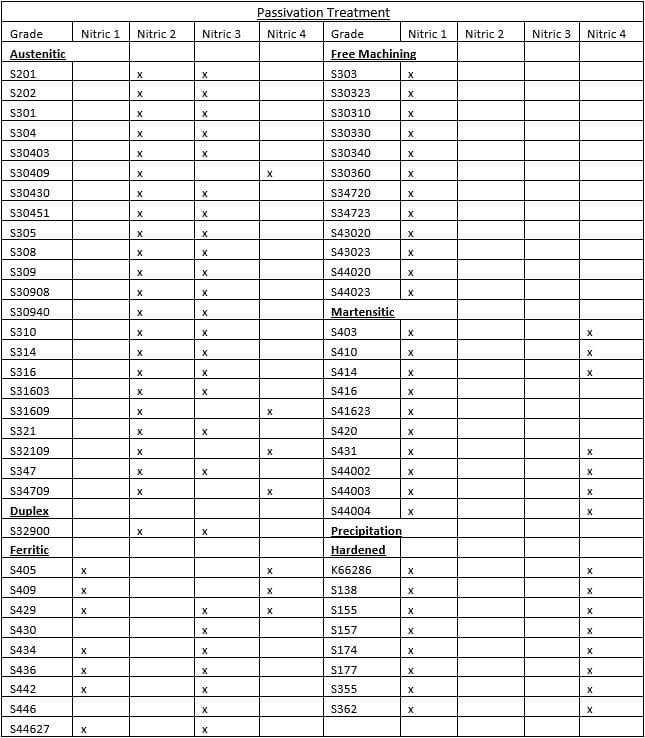
ASTM A967 also offers a very useful reference of stainless steel grades to the recommended method of nitric acid passivation. A summary of this table is provided:
Contamination of passivation chemistry can lead to flash attack of the surface, which produce a heavily etched or darker surface. A common containment that leads to flash attack is chlorides which can come from several sources including dragging in acids or using having chloride in the water. In addition, organic buildup in passivation baths such as the drag-in of machining oils from parts that are not properly cleaned, can lead to flash attack or etching of the stainless steel. As such, regular analytical analysis and maintenance of passivation chemistries is required. Certain passivation methods are also more resistant to flash attacks than others. For nitric acid passivation the baths with increased oxidizing potential are also more resistant to flash attacks. Nitric acid also is more resistant to flash attack compared to citric acid. [1]
Citric Acid Passivation
Citric Acid passivation was developed by Adolf Coors brewing company for the passivation of the inside of beer kegs. It offers an effective alternative to nitric passivation with less handling concerns and is consider environmentally friendly being on the GRAS (Generally Recognized as Safe) list for the FDA making it ideal for food and beverage applications.
When comparing nitric vs citric passivation, citric solutions can effectively passivate a wider range of stainless-steel alloys compared to any one nitric acid passivation solution, allowing for assemblies of several stainless-steel alloys to be passivated.
Passivation chemistries remove free iron from the surface but can also remove some nickel and chromium from stainless steel. Removing nickel and chrome reduces the corrosion resistant material at the surface leaving a thinner oxide layer. Citric acid passivation selectively removes iron over nickel and chromium leaving a thicker corrosion resistant oxide layer than nitric acid passivation [2]
Once of the other advantages of citric acid is the bath formulation can be adjusted to reduce cycle times over nitric acid, allowing for increase throughput and reduced costs of passivation verses that of nitric acid. Cycle times as low as 4 minutes are possible with certain citric acid passivation formulations. A summary of the various citric acid passivation concentrations and times from ASTM A967 are provided below.
- Citric 1: 4-10 w% Citric Acid, 140-160F, 4 Mins minimum
- Citric 2: 4-10 w% Citric Acid, 120-140F, 10 Mins minimum
- Citric 3: 4-10 w% Citric Acid, 70-120F, 20 Mins minimum
- Citric 4: Other combinations of temperature time and concentration of citric acid with or without chemicals to enhance cleaning, accelerants or inhibitors capable of producing parts that pass the specified test requirements.
- Citric 5: Other combinations of temperature time and concentration of citric acid with or without chemicals to enhance cleaning, accelerants or inhibitors capable of producing parts that pass the specified test requirements. Immersion bath to be controlled at pH of 1.8-2.2
Passivation Pretreatment
A universal requirement when comparing nitric vs citric acid passivation is the need for parts to be properly pretreated. For the martensitic grade and precipitation hardened grades of stainless steel that are heat treated, there is a potential for scale on the parts after the hardening process. For machined parts there is cutting fluids and other oils. Finally, for assemblies there is weld scale and heat marks. Any of these scales or oils left on a part lower the corrosion protection of the material and in passivation will inhibit the effectiveness and can damage parts. Scales and oils should be removed before passivation. Oils can simply be cleaned or vapor degreased off parts. While scale needs to be removed either with descaling mineral acids such as hydrochloric acid, or inorganic deoxidizers such as potassium permanganate or with abrasive methods such as media blasting or vibratory polishing. Mechanical scale removal methods are recommended for those parts that require a very uniform surface especially for parts with heat-affected zones such as weldments.
Conclusion
Passivation of stainless steel is a critical component in the manufacturing of stainless-steel components to ensure fully optimized corrosion resistance. There are many different factors when choosing a citric vs nitric passivation method and this article covered some of the basics of choosing a passivation process. For additional information and what process may be right for your application please feel free to contact a member of Advanced Plating Technologies Sales & Engineering group at [email protected] or 414.271.8138.
Blog Authored By: Will T., Process Engineer
References:
[1] Mohr, J. H. (2007, August 1). Making Stainless Steel Stainless. Retrieved from PF Online : https://www.pfonline.com/articles/making-stainless-steel-stainless
[2] R. Kremer, Stellar Solutions, Inc. (2007). Developments In Citric Acid Passivation of Stainless Steel. McHenry : NSF.