By M. Lindstedt
Copper as a Design Material in the Power Market
The power transmission and distribution industry has long utilized copper as the material of choice for designing and manufacturing critical grid components. Copper has excellent thermal and electrical properties that are desirable when transferring and switching very high power loads. In addition, various alloys of copper available provide the machinability and physical/mechanical properties required for most design applications. Copper not only offers outstanding conductivity but its mechanical properties can be enhanced with a range of alloying metals and heat treatments. Grades such as C101 (oxygen free), C110, C145 (tellurium) and C147 (sulfur bearing), C182 (chromium) and C172 (beryllium) are all cuprous alloys that have been utilized within the power transmission and distribution market for decades. All of these grades are readily plated with conductive metals including silver, tin and nickel to enhance the surface conductivity, reduce the formation of insulating oxides and offer enhanced joinability with methods such as brazing and soldering.
Economic Considerations of Copper
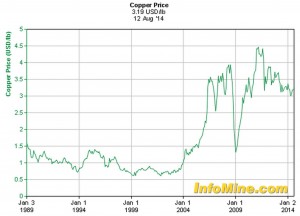
The economic considerations of copper as an engineering material were not a factor throughout most of the 20th century as the price of copper remained reasonably stable. However, the first decade of the 21st century has shown that the growing worldwide demand may outpace supply resulting in significant cost considerations. Figure 1 illustrates the change in the baseline price of the copper commodity over the past 25 years.
Alternatives to Copper for the Power Market
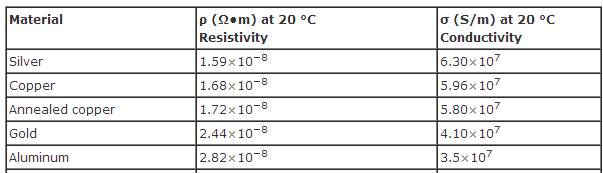
From an engineering perspective, there are few metals that can rival copper for designing critical grid components than handle high current and switching applications. Most metals that can meet the mechanical requirements (e.g. ferrous alloys) offer very poor conductivity and/or thermal characteristics. Other metals that meet the conductivity and thermal requirements are precious metals (e.g. silver and gold) and therefore offer no economic alternative. However, aluminum alloys offer the unique combination of both electrical and mechanical properties to be a potential alternative to copper. Similarly to copper, there is a diversity of aluminum alloys that offer a wide range of design options – form the ultra conductive 1000-series of aluminum to the high-strength 7000-series. Figure 2 lists the electrical conductivity and resistivity of the top four metals. Both aluminum and copper are in the top four and are the only two non-precious metals at the top of the list. As shown in the table, aluminum is actually not far behind gold as a conductor!
Economic Considerations of Aluminum
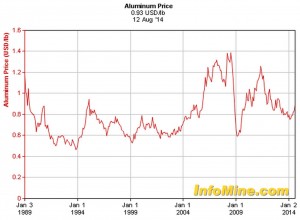
The economic considerations of aluminum show a much more stable history when contrasted to that of copper. In fact current prices for aluminum are below where they were twenty five years ago! Figure 3 shows the cost of aluminum over the past quarter century and illustrate how aluminum is under fewer supply/demand stresses that influence the cost of the commodity. Currently copper is approximately four times the cost of aluminum on the commodity markets. When considering the size of common transmission and distribution switchgear, reclosers and related components; a material savings of this scale offer a huge factor in contrasting design materials.
The “Reactive” Aluminum Consideration
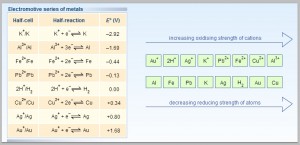
Based solely on the information above, once might ask why aluminum is not more commonly used in lieu of copper within the power transmission and distribution market? Part of the reason is due to the general reactivity of aluminum in most environments. Figure 4 shows a simplified electromotive series for metals including both aluminum and copper:
In the electromotive series metals are compared to hydrogen for reactivity under standard conditions. Those metals with negative potential verses hydrogen are reactive and will readily form oxides. Conversely, metals with a positive potential are more stable and resist oxidation. They are referred to as nobel metals. In comparing aluminum to copper in Figure 4, it is seen that aluminum is a more reactive or less nobel metal than copper. From a design consideration, this means that if aluminum is a component of a bi or tri metal couple it will readily release it electrons and corrode to form its oxide Al2O3. In addition, aluminum is an amphoteric metal meaning that it reacts (is attacked) by both alkaline and acidic environments. It is the reactivity of aluminum that makes it less desirable as an engineering material in most applications unless the surface of the aluminum can be treated to reduce the reactive nature at the surface. Time tested and validated surface coatings such as silver – a nobel metal that does not form oxides under normal conditions – as well as tin – a highly conductive metal that forms a thin and conductive oxide layer – can both be deposited onto aluminum to provide enhanced corrosion resistance and reliable surface conductivity. However, the techniques for plating aluminum are much more complex than that of traditional conductors such as copper. In addition, key surface engineering considerations such as selection of underplates and thickness of the final conductive layers must be reevaluated when aluminum is to replace copper components in an existing design.
Traditional Techniques for Plating on Aluminum
Plating aluminum is a much more involved process than that of traditional engineering materials such as steel and copper. The reason for this lies also with the reactivity of aluminum; aluminum very readily wants to react with the oxygen in the atmosphere forming oxides on the surface. If any plated layers are deposited on top of aluminum oxide, poor adhesion will result. There have been many proprietary systems developed over the years to address the difficulties of plating aluminum including both the Bondal and Alstan processes but each of these processes have limitations when dealing with the range of geometries and aluminum material grades that can be used to replace copper as a design material within the power market. When plating any material the process can be summarized in three basic steps:
- Degrease to remove dirt, oils and grease from the surface
- Deoxidize the surface to remove metal oxides
- Plate
These simple steps are basically followed when plating traditional materials such as copper and steel. However, when plating aluminum, the reactive nature of the metal reforms oxides immediately after the deoxidizing step and prior to plating. As a result, more complex plating processes had to be developed to address the rapid oxidation of the surface. The traditional zincate process was developed circa 1950 to address the affinity of aluminum to form oxides. A traditional zincate process adds two steps between the deoxidation and subsequent plating steps in which a zinc immersion deposit is applied to the aluminum followed by an electrolytic copper strike. The unique chemistry of the zincate process has the ability to remove any residual oxides that formed after the deoxidation step and apply a thin coating of zinc. The zinc layer essentially “shrink wraps” the aluminum to prevent further oxidation from occurring prior to the subsequent copper strike. The key to a successful traditional zincate process is for the zinc to be thin, dense and adherent to the aluminum such that the following plating step will adhere to the zinc and in turn to the aluminum. The Bondal and Alstan processes built upon the zincate process to improve the overall bond. The Bondal process is a proprietary zincate process in which a quad metal zincate (zinc/copper/nickel/iron) is used to bond to the aluminum. It is held that this process forms a very tight zincate receptive to subsequent plating. The Alstan is a proprietary process in which a stannate activation is used in lieu of a zincate step. The stannate activation applies a thin layer of immersion tin on the surface which is subsequently plated over with a bronze (tin/copper) alloy deposit. It is held that some of the tin from the stannate activation step is incorporated into the bronze deposit thereby promoting adhesion.
Limitations of Traditional Aluminum Plating Processes
Any of the above traditional methods for plating on aluminum are limited by two primary factors:
- They rely on immersion deposits to bond subsequent plating layers to the surface
- They employ electrolytic plating strikes after the immersion deposits
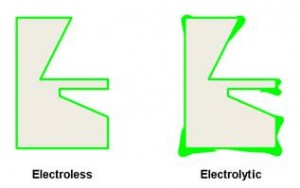
The adhesion of immersion deposits is tenuous in a production job shop environment. Variability in aluminum grade compositions, time, temperature and zincate concentration all lead to inconsistencies in grain structure and bond of an immersion deposit. Double zincate processes as well as dual and quad metal zincates all attempt to address this shortcoming of traditional aluminum plating to achieve the most dense and thin immersion layer possible. However, all of these processes rely on the adhesion of the immersion layer to anchor subsequent plating to. Electrolytic plating strikes used after traditional or proprietary Alstan and Bondal processes are limited by part geometry. As part’s shape becomes more complex the ability of the electrolytic bronze of copper strikes to fully cover the zincate layer becomes more tenuous. Electrolytic plating processes by their very nature do not apply uniformly to the surface of apart. As the geometry of the part becomes more complex (e.g. counter bores, thru-holes, blind holes, recesses, high/low points) the ability of an electrolytic strike to consistently cover those features decreases. The Figure 5 illustrates the difference in deposits between an electrolytic (right) and electroless (left) process:
If the zinc from a traditional zincate process is not completely covered by the subsequent cyanide copper strike the zinc will be attacked in subsequent plating steps resulting in pitting, blisters and failed adhesion. As the part geometry becomes more complex (current exchange housings, junction bushings with blind holes, tubular components, wave guide housings) the shortcomings of using an electrolytic strike after an immersion deposit can become very apparent especially in a high volume production scenario.
Advanced Techniques for Plating on Aluminum

APT-AL Process: Pretreatment

APT-AL Process: Plated Layers
After a properly tailored pretreatment, the APT-AL process utilizes a modified double zincate process followed by advanced electroless nickel chemistries adapted for aluminum to provide a consistently bonded nickel strike independent of part geometry. The key to the APT-AL process is that the zincate layer is completely removed in the following electroless nickel strike. As such, the bond of the zincate does not impact the bond of the final plated layers since the electroless nickel bonds directly to the aluminum with no immersion deposits in between. 
Summary
As the economic pressures of copper continue to increase over the upcoming decade, aluminum stands as the most viable alternative for most power grid components. However, the reactive nature of aluminum demands that the surface be properly engineered to ensure proper and consistent functionality within the diverse range of field environments. The entire surface finish including pretreatment, strikes, underplates and topcoats need to be evaluated to ensure that aluminum components will function as designed over the lifespan of the product. By utilizing the most advanced techniques for plating aluminum, the design engineer can have the assurance that the final deposit of silver, tin or nickel will remain adherent and provide the surface properties required for safe, reliable and efficient transfer of energy.