One of the greatest shortcomings for the longevity of copper, brass, or even stainless steel contacts is corrosion of the base material. This issue is a greater problem in harsh or extended duty contacts which is why gold plating is preferred for these applications.
Gold plating deposits a noble gold layer that will not corrode or form oxides or compounds even at elevated temperatures or when exposed to highly corrosive environments. An added benefit is that gold is a better electrical and thermal conductor than the many of the base materials contacts are fabricated from.
Corrosion & Oxidation of Contacts or Terminals
Most gold connectors and contacts are not made from pure gold but are rather fabricated from a base metal such as C172 or C173 beryllium copper, C510 phosphor bronze, C360 leaded brass or austenitic stainless steel. This is for obvious cost reasons but also to provide a foundation material with the strength and spring temper that the design requires. Since these base materials are not precious metals, they are subject to corrosion which forms compounds on the surface such as oxides, sulfates or sulfides.
austenitic stainless steel. This is for obvious cost reasons but also to provide a foundation material with the strength and spring temper that the design requires. Since these base materials are not precious metals, they are subject to corrosion which forms compounds on the surface such as oxides, sulfates or sulfides.
Since these compounds are not conductive metals, they interfere with the transfer of current or signals through the conductor. Corrosion and the formation of these compounds occur mainly when the base metal is exposed to the atmosphere or galvanic corrosive effects from contact with other metals.
Copper and Copper Alloys (e.g. C101 Oxygen Free Copper, C110 Copper, C172 or C173 Beryllium Copper, C510 Phosphor Bronze) corrodes when exposed to elements in the environment including oxygen and sulfur. Copper corrosion products such as cupric or cuprous oxide as well as copper carbonate, sulfide or sulfate can appear brown, blue or green (patina) depending on the compound formed and the pH of the environment.
The Statue of Liberty is a great example of the patina that is formed when copper is exposed to the acidic environment of New York’s acid rain. The compounds that form on copper becomes a protective film that slow the rate of future corrosion. The negative effect of this is that the copper compounds formed are not conductive and degrade the performance of the electrical contact or connector.
Brass (e.g C260 Brass, C360 Free Machining Brass) being an alloy of copper and zinc, oxidizes and corrodes very similarly to copper. It also corrodes because of the high amount of zinc that is alloyed with the copper. The addition of the zinc makes the brass less corrosion resistant than copper and more susceptible to both corrosion and loss of zinc or dezincification. In either scenario the brass will become less conductive and will not be a good electrical connector.
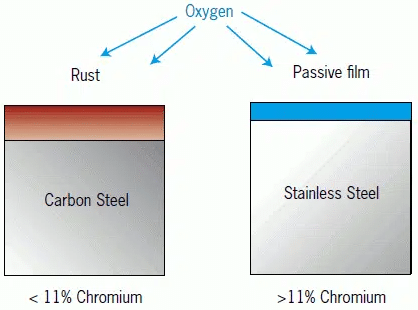
Stainless Steel (e.g. 304 or 316 Stainless Steel or 303 or 416 Free Machining Stainless Steel) is often thought of as being free from corrosion. However, stainless steels are very susceptible to attack from chlorides found in bleach or salt water and can form traditional rust readily in these environments.
In addition, in less aggressive environments, stainless steel naturally forms a thin nickel/chrome oxide on the surface that protects the steel from further oxidation. However, this film like all oxides is less conductive and will increase the contact resistance of the terminal or contact.
Underplate Role in Minimizing Corrosion of Gold Plated Contacts
Gold plating will not corrode but having a layer of gold on the parts will not stop the substrate from corroding or oxidizing. This is due to the fact that thin layers of plated metals have small pores which allow for corrosion to propagate through.
To minimize gold plating corrosion through the plating there are several design factors that must be taken into consideration. The first is the thickness of the plating, in most scenarios the thicker the gold plating the better the corrosion resistance will be. But the second and more important factor is the porosity of the plating.
Although the thickness of gold plating is key to making contacts have more corrosion protection, there are limitations to the gold thickness primarily due to cost. In addition a higher gold thickness, nickel plating is used to increase overall plating thickness and make the parts more corrosion resistant. It is the combination of gold plating and nickel plating that can help make gold plated electrical contacts and connectors corrosion resistant.
Plating Porosity is a major factor in corrosion resistance of any gold plated parts. At thin thicknesses below 0.0005” per side, most plated metals have an intrinsic porosity that allows corrosive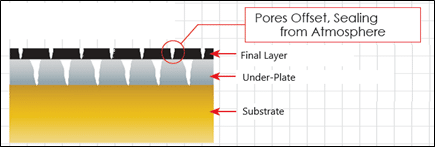
Each plated layer has a unique porosity similar to a fingerprint, as more layers are added it is less likely for the pores to reach the base material. This is due to the fact that pores are concentrated at the grain boundaries and each plated layer has a unique grains structure. Adding layers adds the complexity of the path from the substrate to the surface for a continuous pore to exist.
A tangible example of this phenomena is pouring water through a screen. The water will not pour thru the screen as fast as pouring water straight from a pitcher. Likewise, as more screens are added, the water will flow more slowly. The rate of the water flow through the screens can be thought of the rate at how quickly corrosion will occur in plated layers.
As the thickness of the plated layers are increased, the pore size and density in the screen becomes smaller and smaller. By plating thicker layers to reduce the pore size and adding more layers (screens) eventually, the water flow can be stopped. This condition is what is referred to as a pore-free barrier coating.
Nickel Plating is a common underplate for gold plated contacts because it is a hard metal that is load-bearing, which is useful when both the base material and the final plating layer are soft metals (such as a copper part and gold plating).
Nickel plating prevents parts from corroding by becoming a barrier between the substrate and the environment and it works by physically stopping the moisture and air from getting to the copper, brass, or steel. Nickel also can work as a diffusion barrier to prevent the copper from migrating through the gold to the surface which is undesirable since the copper could then oxidize or corrode on the surface.
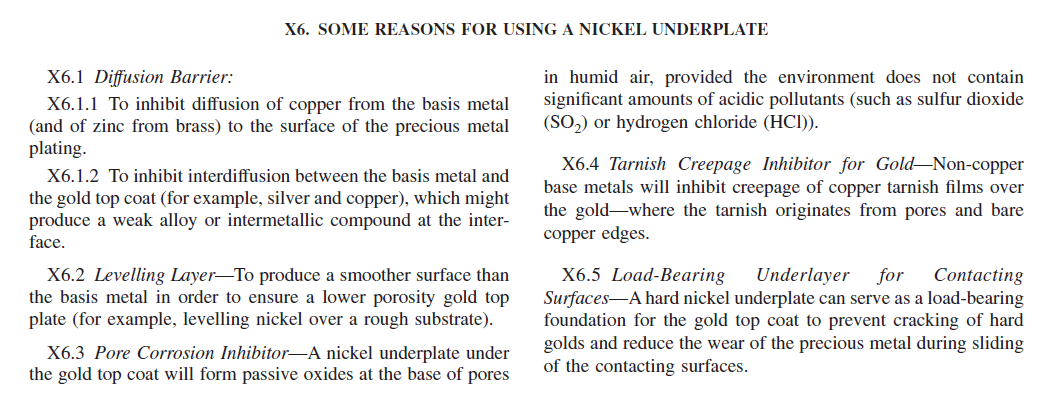
ASTM B488, Standard Specification for Electrodeposited Coatings of Gold for Engineering Uses, summarizes the reasons for utilizing a nickel underplate in Appendix X6 which is provided in Figure 2 below:
Copper Plating is an additional layer that can be added to gold and nickel plated contacts in order to increase the overall corrosion resistance. Figure 2 illustrates the porosity of a part that has a gold over nickel plating (top) and one with gold over nickel over a copper subplate (bottom). The reduction in pore density is significant which directly correlates to better corrosion resistance.
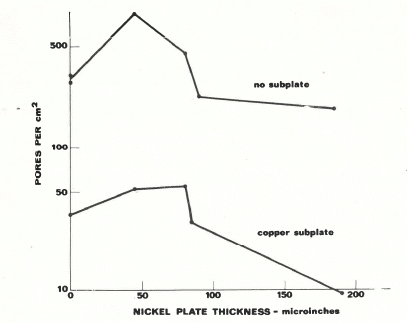
Figure 2: Pore Density of a Gold and Nickel Plated Contact with a Copper Underplate (lower plot) and Without a Copper Underplate (upper plot)
The reduction in porosity is due to the additional layer of plating pores that would need to line up in order for the moisture to reach the substrate. However, copper is not recommended to be the penultimate layer before the final gold since it can both oxidize through the gold as well as form a gold/copper eutectic through solid state diffusion. This will not only reduce the effective gold thickness but will also form a weak eutectic that can reduce adhesion and conductivity.
Gold Plating Specifics to Optimize Corrosion Resistance of Contacts
Gold Plating is the final deposit in connectors and contacts due to its outstanding electrical properties and noble properties. Because of gold’s inert nature it will not corrode or oxidize. However, the above noted underplate porosity and thickness characteristics need to be taken into consideration to ensure the gold plated contact is sufficiently robust for the intended application.
Duplex Gold Plating is a special type of plating gold that combines two different types of gold – soft gold (99.9% pure) and hard gold (99.0-99.7% pure). This is done to decrease the effective porosity in overall gold plated layers since the two gold layers have unique grain structures which do not align. Which gold layer should be the final or ultimate layer is a function of the part use as summarized below:
· Static Connectors: Gold plated static connectors are connectors that, when installed, make a connection with something that is intended to stay in contact with it. A few examples of this are grounding nuts or studs, fixed contacts or solder pads. Due to the small amount of movement the hardness of the gold plating is not as important and soft gold is the preferred final or ultimate layer.
· Dynamic Connectors: Gold plated connectors that make repeated connection with something are considered dynamic. A few examples of this are male/female pins/sockets and battery contacts. Since the wear of these connectors is of principle concern, hard gold is the preferred final or ultimate layer. As such, with most dynamic connector duplex gold applications, the hard gold should be the outermost layer.
Gold Thickness is a key characteristic in the corrosion resistance of gold plated connectors. Since the cost of gold plating is directly proportional to gold thickness, the target thickness should be carefully considered and evaluated.
Figure 4 below details four common connector thicknesses and common applications for each. This also illustrates how higher thickness correlates to improved corrosion performance. As noted in the table, a thin layer of gold is sufficient for static connections in controlled environments. However, as the wear resistance and/or corrosion requirements of the application increase, so must the gold thickness as well as the thickness of the respective underplates.
Figure 4 below: Thickness of Gold Plating Based on End Use and Environment
| Common Thicknesses of Gold | Relevant ASTM B488 Class | Relevant MIL-G-45204 Class | Applications |
|---|---|---|---|
| 10µin, 0.25µm | Class 0.25 | N/A | Suitable for static connections in controlled environments without cyclical use. Good for solderability and wire bonding (10-20uin) |
| 30µin, 0.75µm | Class 0.75 | Class 0 | Good for connections that may be soldered. Contacts can be exposed to moderate environments and wear cycles but not high cycle or chemical attack. |
| 50µin, 1.25µm | Class 1.25 | Class 1 | Suitable for connections that may be soldered, exposed to more aggressive environments, offers superior protection against wear in moderate to high cycles. |
| 100µin, 2.5µm | Class 2.5 | Classes 2 | Not recommended for connections that may be soldered, offers excellent protection against corrosive environments and durability high wear applications |
Conclusion
Gold plating offers many advantages for connectors and terminals including excellent electrical and thermal conductivity and reliable contact resistance for electronic applications and more. However, gold plated connectors need to be designed properly to meet the corrosion resistance of the intended application or the parts will not perform reliably.
All base materials used for making contacts or terminals will form compounds or oxides which can migrate to the surface of the gold plated layer increasing the contact resistance. By using multiple plated layers including copper, nickel, gold and even duplex gold plating the overall porosity of the deposit is decreased thereby increasing corrosion resistance. Increasing the thickness of each of the plated layers helps reduce the pores in the plating of the parts which makes it harder for the atmosphere and moisture to get to the substrate.
The above information is provided as a general guide for engineering a finish for a specific contact or connector. There are many additional considerations specific to each plating application that are beyond the scope of this article.
Advanced Plating Technologies an ISO 9001:2015 gold plating company offers extensive surface engineering support for gold plating services or other applications. Reverse engineering of existing or failed applications and components is available to provide design assistance. Feel free to contact a member of APT’s technical sales team for further assistance at [email protected] or 414.271.8138.
Blog authored by Dominic Scardino, Estimating Engineer
References:
- Reid, F. H., & Goldie, W. (1987). Gold Plating Technology (Reprint ed.). Amer Electroplaters Soc.
- (2021, September 28). Protecting electrical connectors from water ingress with Nyogel 760G. Newgate Simms Tech Support. https://support.newgatesimms.com/protecting-electrical-connectors-from-water-ingress-with-nyogel-760g/
- Ignition Switch, Repairing electrical parts, MGA. (2014). MGAGuru. http://mgaguru.com/mgtech/electric/et126.htm
- (2018, June 11). How Much More Chromium Does D2 Need to be Stainless? Knife Steel Nerds. https://knifesteelnerds.com/2018/06/11/how-much-more-chromium-does-d2-need-to-be-stainless/
- Grain size analysis in copper. (2019, July 26). Clemex. https://clemex.com/analysis/grain-size-analysis-2/