All metals, with the exception of the precious metals, will oxidize when exposed to oxygen and an electrolyte (i.e. atmospheric moisture). It is a chemical reaction of the metal surface with the oxygen present in the air that causes some of the metal to corrode (or oxidize) and form the respective metal oxide on the surface. In some metals such as steel, the corrosion products formed are very visible and loose. Everyone has observed the red color of iron oxide (rust) seen on improperly protected steel products. The red rust formed is generally scaly and loose and easily falls away exposing more and more basis material to the environment. However, metals such as stainless steel (steel with added nickel and chromium) oxidize as well. The difference is that the nickel and chromium oxides formed are a more uniform and tenacious oxide layer that protects the underlying material by “sealing” the surface from further oxidation once formed.
In addition to the surface oxidation that occurs on individual metals, any two dissimilar metals placed in contact with one another with an electrolyte (such as atmospheric moisture or water) will form a corrosion cell. This is the very basis of batteries used in everyday products. One of the two metals in contact will corrode in preference to the other and form that metal’s respective oxide. Which metal corrodes is based on what chemists call the electromotive series of metals. The selection of what plated layer to use is an important one since electroplating in its very essence is the process of placing two dissimilar metals in contact with one another. The plated layer (or layers) can either be an anodic coating (coating corrodes in preference to the substrate) or cathodic coating (substrate corrodes in preference to the plated layer). Whether a coating is an anodic or cathodic coating greatly impacts how the finishing system will perform once in service and there are advantages and disadvantages to each.
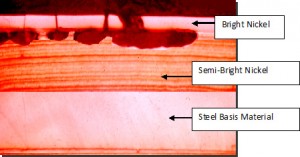
Figure F.1 shows a cross section of a two-layer (duplex) nickel plated product as an example of a cathodic coating. The corrosion occurs between the two dissimilar nickel layers (bright nickel and semibright nickel) which forces the corrosion to propagate laterally. The use of two layers of dissimilar nickel helps prevent the galvanic attack from occurring between the basis steel and the plated layer.
Have Questions?